Do you seek for 'molecular geometry assignment answers'? All material can be found on this website.
Table of contents
- Molecular geometry assignment answers in 2021
- A molecule with the formula beh2 has a shape.
- Sp3-orbitals bent tetrahedral trigonal bipyramidal
- Molecular geometry answer key pdf
- Molecule shapes worksheet answer key
- Molecular geometry answer key
- Two molecules have the same molecular geometry
- If a molecule has four hybrid sp3 orbitals, it can be concluded that the molecule has a
Molecular geometry assignment answers in 2021
 This image representes molecular geometry assignment answers.
This image representes molecular geometry assignment answers.
A molecule with the formula beh2 has a shape.
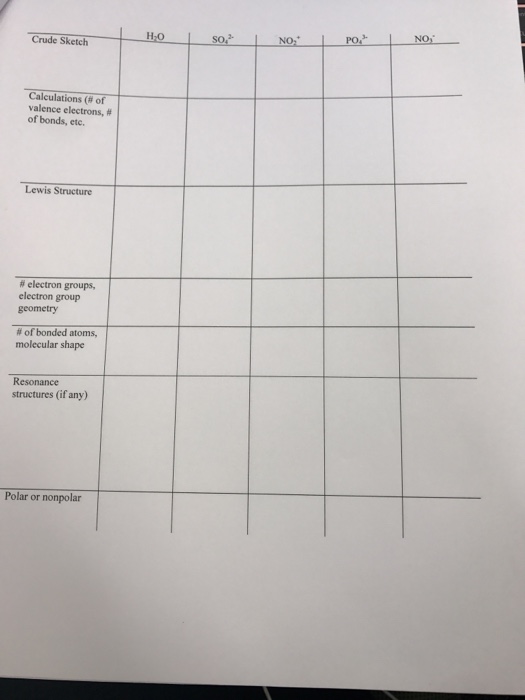 This picture representes A molecule with the formula beh2 has a shape..
This picture representes A molecule with the formula beh2 has a shape..
Sp3-orbitals bent tetrahedral trigonal bipyramidal
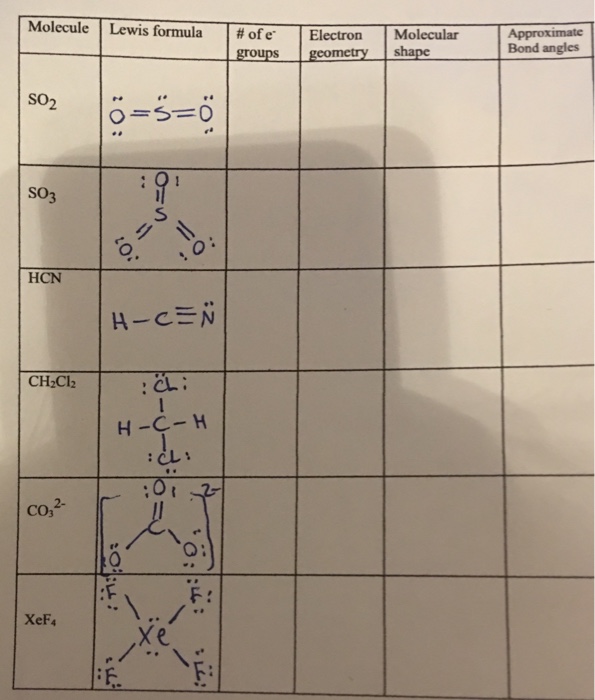 This picture shows Sp3-orbitals bent tetrahedral trigonal bipyramidal.
This picture shows Sp3-orbitals bent tetrahedral trigonal bipyramidal.
Molecular geometry answer key pdf
 This picture illustrates Molecular geometry answer key pdf.
This picture illustrates Molecular geometry answer key pdf.
Molecule shapes worksheet answer key
 This picture demonstrates Molecule shapes worksheet answer key.
This picture demonstrates Molecule shapes worksheet answer key.
Molecular geometry answer key
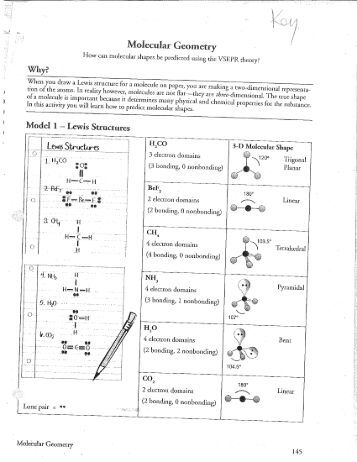 This image demonstrates Molecular geometry answer key.
This image demonstrates Molecular geometry answer key.
Two molecules have the same molecular geometry
 This picture shows Two molecules have the same molecular geometry.
This picture shows Two molecules have the same molecular geometry.
If a molecule has four hybrid sp3 orbitals, it can be concluded that the molecule has a
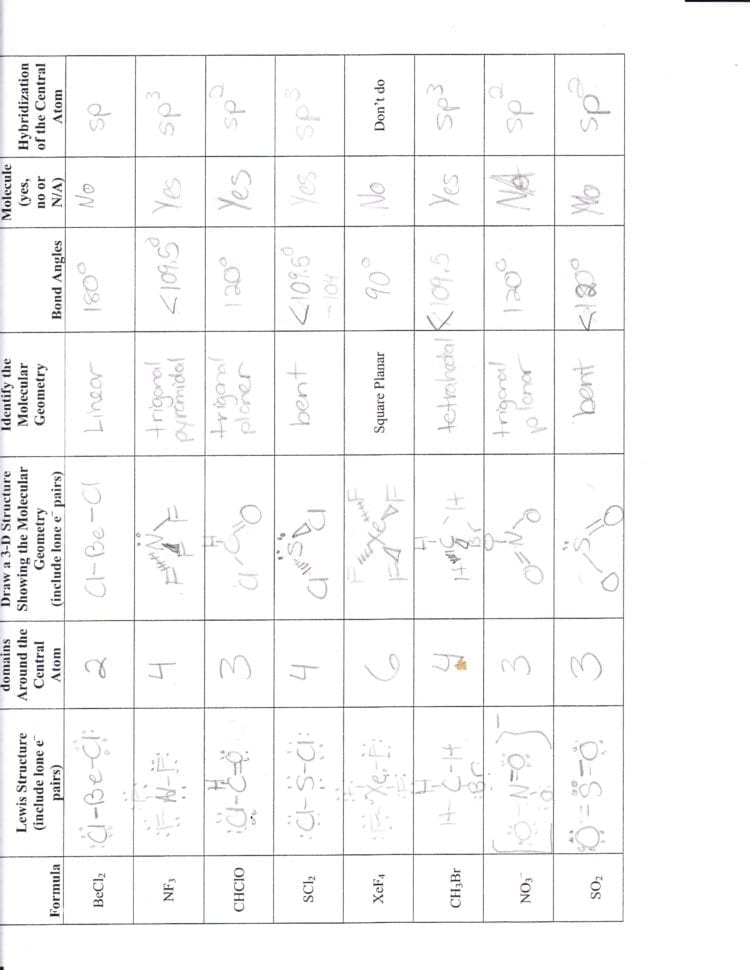 This image representes If a molecule has four hybrid sp3 orbitals, it can be concluded that the molecule has a.
This image representes If a molecule has four hybrid sp3 orbitals, it can be concluded that the molecule has a.
How to write out the molecular geometry of a molecule?
A molecule that has a bent shape and a trigonal-planar electron domain shape _____ lone pairs. Write in the name of the molecular geometry for each molecule described below. A molecule with two atoms and no lone pairs around the central atom has a _____ shape.
How to predict the electron domain of a molecule?
Use the Lewis structure to predict the electron domain geometry of each molecule. Use the Lewis structure to predict the molecular geometry of each molecule. Use the Periodic Table to determine the shape of the molecule represented by the following formulas.
How is the bonding geometry of the H2O molecule described?
Therefore, although the oxygen atom is tetrahedrally coordinated, the bonding geometry (shape) of the H 2 O molecule is described as bent. The effect of the lone pair on water: Although the oxygen atom is tetrahedrally coordinated, the bonding geometry (shape) of the H2O molecule is described as bent.
Which is type of Hybridization leads to bent molecular geometry?
The hybrid orbitals of a molecule are shown in the figure. one s orbital and one p orbital. Which type of hybridization leads to a bent molecular geometry and a tetrahedral electron domain geometry? Consider the Lewis dot structures below, which represent the valence electrons of carbon (C) and chlorine (Cl).
Last Update: Oct 2021