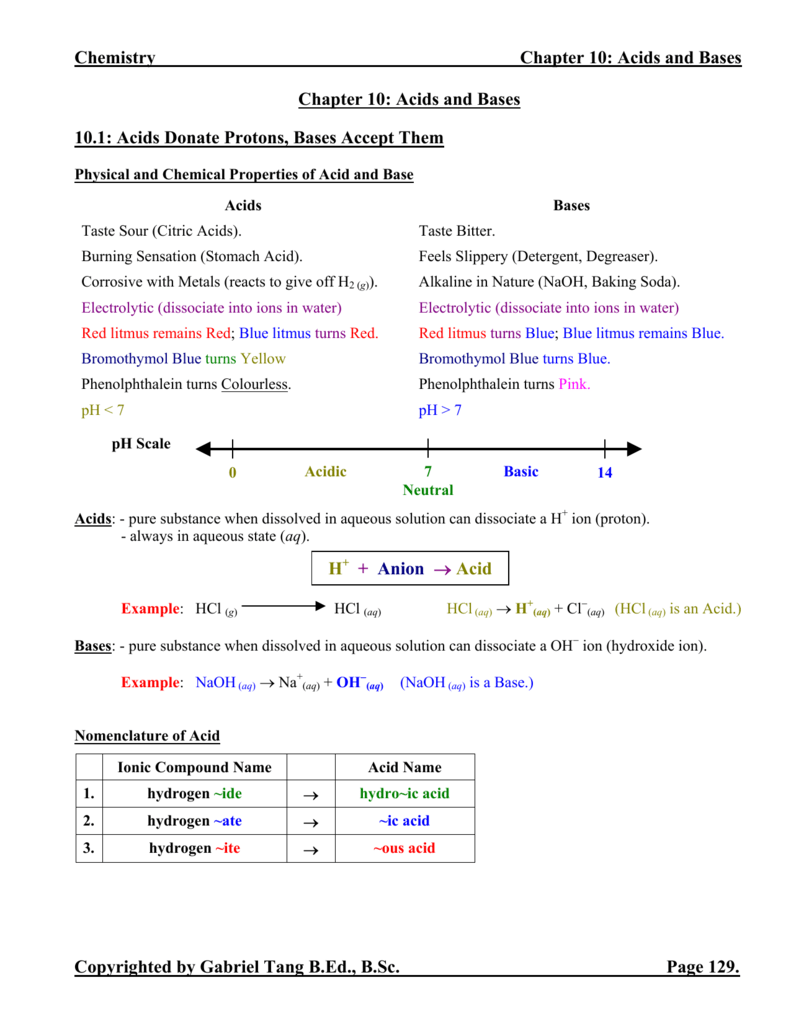
Are you interested in finding 'ch 14 outline acid and bases'? All the details can be found on this website.
Table of contents
- Ch 14 outline acid and bases in 2021
- Chapter 14 acids and bases answer key
- Chapter 14 acids and bases section 3 answer key
- Ap chemistry chapter 15 notes
- Chapter 15 acid base equilibria
- How many stages of ionization does phosphoric acid go through?
- Chapter 14 acids and bases quizlet
- Chapter 14 review acids and bases
Ch 14 outline acid and bases in 2021
 This picture shows ch 14 outline acid and bases.
This picture shows ch 14 outline acid and bases.
Chapter 14 acids and bases answer key
 This image shows Chapter 14 acids and bases answer key.
This image shows Chapter 14 acids and bases answer key.
Chapter 14 acids and bases section 3 answer key
 This image shows Chapter 14 acids and bases section 3 answer key.
This image shows Chapter 14 acids and bases section 3 answer key.
Ap chemistry chapter 15 notes
 This picture illustrates Ap chemistry chapter 15 notes.
This picture illustrates Ap chemistry chapter 15 notes.
Chapter 15 acid base equilibria
 This picture illustrates Chapter 15 acid base equilibria.
This picture illustrates Chapter 15 acid base equilibria.
How many stages of ionization does phosphoric acid go through?
 This picture representes How many stages of ionization does phosphoric acid go through?.
This picture representes How many stages of ionization does phosphoric acid go through?.
Chapter 14 acids and bases quizlet
 This image demonstrates Chapter 14 acids and bases quizlet.
This image demonstrates Chapter 14 acids and bases quizlet.
Chapter 14 review acids and bases
 This image demonstrates Chapter 14 review acids and bases.
This image demonstrates Chapter 14 review acids and bases.
Which is NCERT solution for Chapter 2 acid bases and salts?
NCERT Solutions for Class 10 Chapter 2 Acids, bases and salts are prepared to help students in their exam preparation. This solution provides you with answers to the questions provided in the NCERT Class 10 textbooks.
How to determine if a substance is an acid or base?
To determine whether a substance is an acid or a base, before and after the reaction, count the hydrogens on each substance. If the number of hydrogens decreases this substance is the acid (donates ions of hydrogen). If the number of hydrogen has increased this substance is the basis (accepts ions of hydrogen).
What can you learn from acid bases and salts?
In this chapter, students will learn about the nature and behaviour of Acids, bases and salts. This chapter mainly describes the chemical nature of acids, bases and salts, their reaction with metals, non-metals and with each other. This chapter is quite interesting as it involves many practical experiments to help you learn the basics.
How are acids and bases defined in Bronsted theory?
1 The Bronsted-Lowry theory defines an acid as a donor of protons. 2 A base is defined as a proton acceptor (or H+ion acceptor) by this theory. 3 Bronsted acids undergo dissociation to yield protons and therefore increase the concentration of H+ions in the solution. More items...
Last Update: Oct 2021